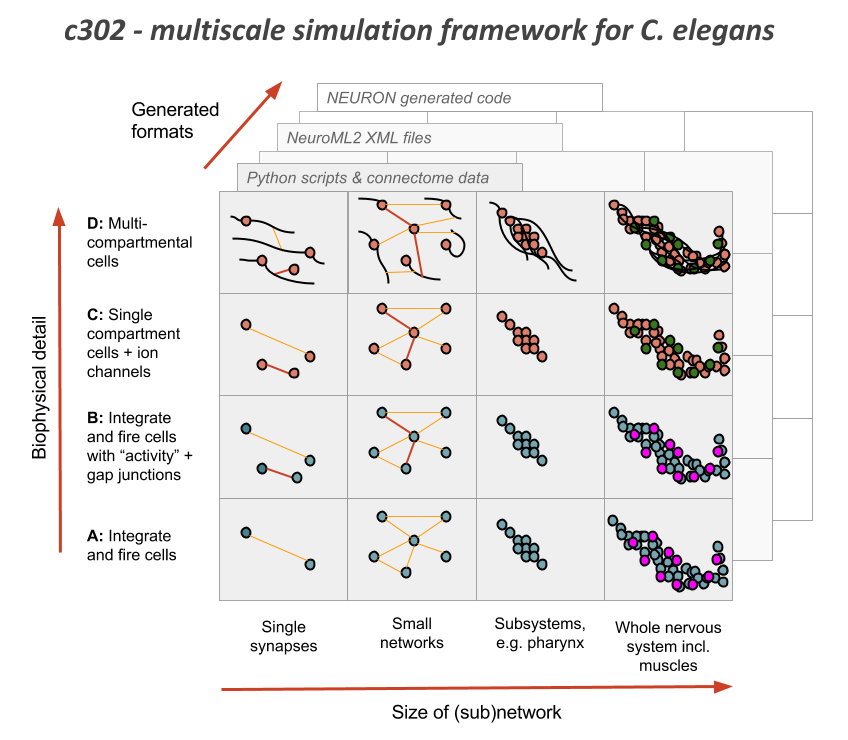
c302 is a framework for generating network models in NeuroML 2 based on C. elegans connectivity data. It is primarily intended as a way to generate neuronal networks at multiple levels of detail for the OpenWorm project. To see how c302 relates to other OpenWorm subprojects click on the image below:
It uses information on the synaptic connectivity of the network (from here) and uses libNeuroML to generate a network in valid NeuroML, which can be run in jNeuroML or pyNeuroML.
The c302 paper has recently been published!
c302: a multiscale framework for modelling the nervous system of Caenorhabditis elegans Padraig Gleeson, David Lung, Radu Grosu, Ramin Hasani, Stephen D. Larson, Phil. Trans. R. Soc. B 2018 373 20170379; DOI: 10.1098/rstb.2017.0379.
The full set of dependencies for c302 can be installed with the following (see also the Travis-CI script):
git clone https://github.com/openworm/c302.git
cd c302
python setup.py install
This will install c302 as well as all dependencies, including pyNeuroML and PyOpenWorm.
To ensure everything is set up correctly try:
-
Regenerate the NeuroML & LEMS files for one instance of the model:
python c302/c302_Pharyngeal.py B # generate pharyngeal network (see fig. above) using parameter set B -
Run a simulation with pyNeuroML:
pynml examples/LEMS_c302_B_Pharyngeal.xml
To test all of the working features of the framework run test.sh:
./test.sh # or ./test3.sh if you use Python 3
There are a number of example models included with the standard distribution. These consist of: A) generated NeuroML 2 network description file (example), containing the definitions of the cells to use (e.g. iafCell for an integrate and fire cell), any inputs (e.g. pulseGenerator) as well as the populations, projections and inputLists contained within the network (for a full description of the NeuroML elements see here); and B) a LEMS simulation file (example) describing how long to simulate, the timestep and what to plot/record.
# generate 2 neurons & 1 muscle with current inputs using parameter set A
pynml examples/LEMS_c302_A_IClamp.xml
# generate full scale network using parameter set C
pynml examples/LEMS_c302_C_Full.xml
# generate pharyngeal network using parameter set B
pynml examples/LEMS_c302_B_Pharyngeal.xml
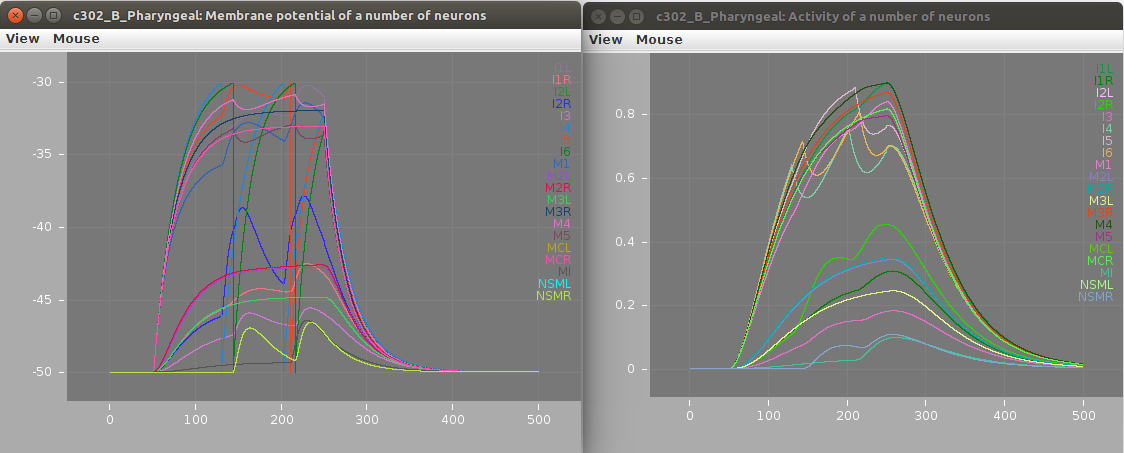
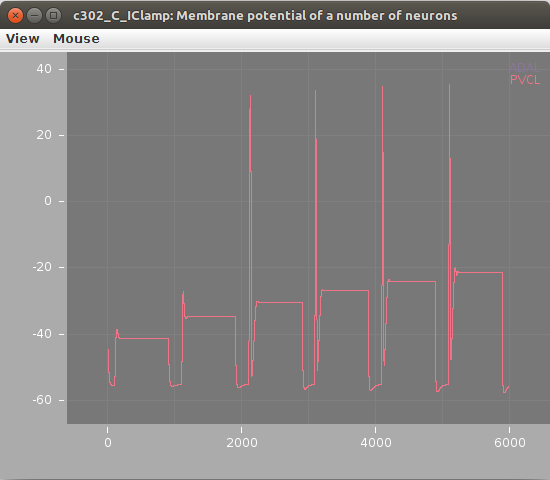
Screenshots of a simulation with pyNeuroML of c302_B_Pharyngeal are shown below (left: membrane potential of 20 cells, right: "activity" of 20 cells - a value from 0-1 showing time smoothed activity of each cell):
The models can also be run using the Neuron simulator. This should be installed as outlined here.
cd examples
pynml LEMS_c302_A_IClamp.xml -neuron # Generate the Neuron files (Python/hoc/mod)
nrnivmodl # Compile the mod files (used for cell/ion channel definitions)
nrngui LEMS_c302_A_IClamp_nrn.py # Run the main Python file for the simulation using Neuron
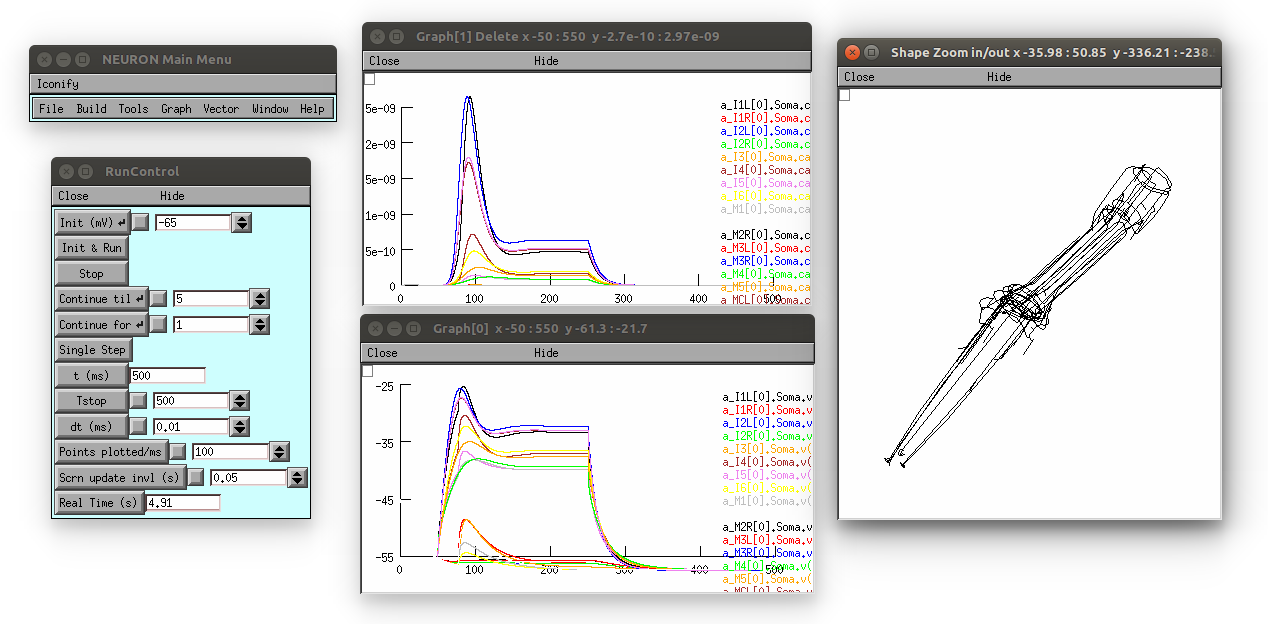
Note: models with the D parameter set can only be run using Neuron (not pyNeuroML), simnce they consist of multicompartmental Neurons, e.g.
pynml LEMS_c302_D_Pharyngeal.xml -neuron
nrnivmodl
nrngui LEMS_c302_D_Pharyngeal_nrn.py
This produces the following (graph on top is [Ca2+], bottom is membrane potential; 3D view on right can be produced by selecting in the Neuron main menu: Graph -> Shape plot)
The c302 command line utility can be used to generate customised networks of varying size, with different cells stimulated, of varying duration from the command line:
c302 MyNetwork parameters_C -cells ["AVBR","VD3"] -cellstostimulate ["AVBR"] -paramoverride {"unphysiological_offset_current":"2.9pA"} -duration 300
This will create a NeuroML 2 file and a LEMS file to execute it, containing 2 cells, stimulating 1 of them, and with a duration of 300 ms. It can be run with:
pynml LEMS_MyNetwork.xml
To see the structure of the network, use pyNeuroML:
pynml MyNetwork.net.nml -graph 4c # Try other options like 1, 2f, 5c for varying levels of detail
More options for using the c302 command can be found with
c302 -h
To investigate how the behaviour of a model changes when parameters are varied, it is possible to change the parameters in the parameters_X.py files and regenerate.
For example in parameters_C.py there are lists of parameters like:
self.add_bioparameter("muscle_leak_cond_density", "5e-7 S_per_cm2", "BlindGuess", "0.1")
self.add_bioparameter("neuron_leak_cond_density", "0.005 mS_per_cm2", "BlindGuess", "0.1")
self.add_bioparameter("leak_erev", "-50 mV", "BlindGuess", "0.1")To change the model behaviour alter one of these values, e.g.
self.add_bioparameter("neuron_leak_cond_density", "0.02 mS_per_cm2", "BlindGuess", "0.1")and look at the behaviour afterwards (note the package needs to be reinstalled)
sudo python setup.py install # reinstall package after change
python c302/c302_IClamp.py C # regenerate c302_C_IClamp
pynml examples/LEMS_c302_C_IClamp.xml # run simulation
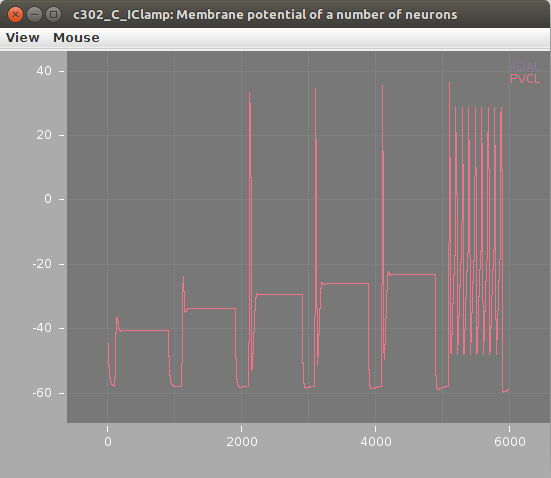
The plots below show the neuron's membrane potential on application of 6 increasing pulses of current before (left) and after (right) the change, indicating how increasing the leak conductance removes the spiking:
The structure of the model generated can be altered by modifying the NeuroML model returned in the c302_XXX.py script. As an example, say we want to add a sine wave current to the Muscles network (specified by c302_Muscles.py), as opposed to the steady current clamp input. This can be achieved by setting the "unphysiological_offset_current" to zero:
params.set_bioparameter("unphysiological_offset_current", "0pA", "Disabling offset current", "0")and (after calling c302.generate()) adding the new NeuroML element for the current and adding the input to a cell:
# Import from libNeuroML
from neuroml import SineGenerator, InputList, Input
import neuroml.writers as writers
# Create the sine wave current generator & add to NeuroML document
sw_input = SineGenerator(id='NewSineWaveInput',
delay='100ms',
phase='0',
duration='800ms',
amplitude='4.5pA',
period='200ms')
nml_doc.sine_generators.append(sw_input)
# Which cell to stimulate
cell = 'AVBL'
# create an InputList and add one Input to that cell
input_list = InputList(id="Input_%s_%s" % (cell, sw_input.id), component=sw_input.id, populations='%s' % cell)
input_list.input.append(Input(id=0, target="../%s/0/GenericNeuronCell"%cell, destination="synapses"))
nml_doc.networks[0].input_lists.append(input_list)
# Write over network file created already...
nml_file = target_directory+'/'+reference+'.net.nml'
writers.NeuroMLWriter.write(nml_doc, nml_file)These changes are made in c302_MusclesSine.py) and can be run with:
python c302/c302_MusclesSine.py C
pynml examples/LEMS_c302_C_MusclesSine.xml
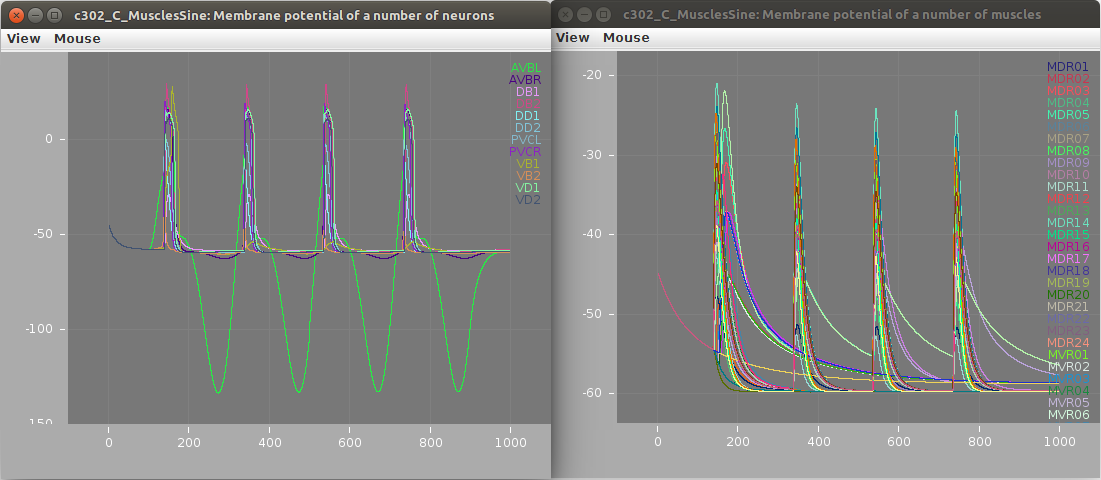
Membrane potential of neurons (left; stimulated AVBL cell in green) and muscles (right) shown below:
Other types of NeuroML input elements are defined here and examples are shown here.
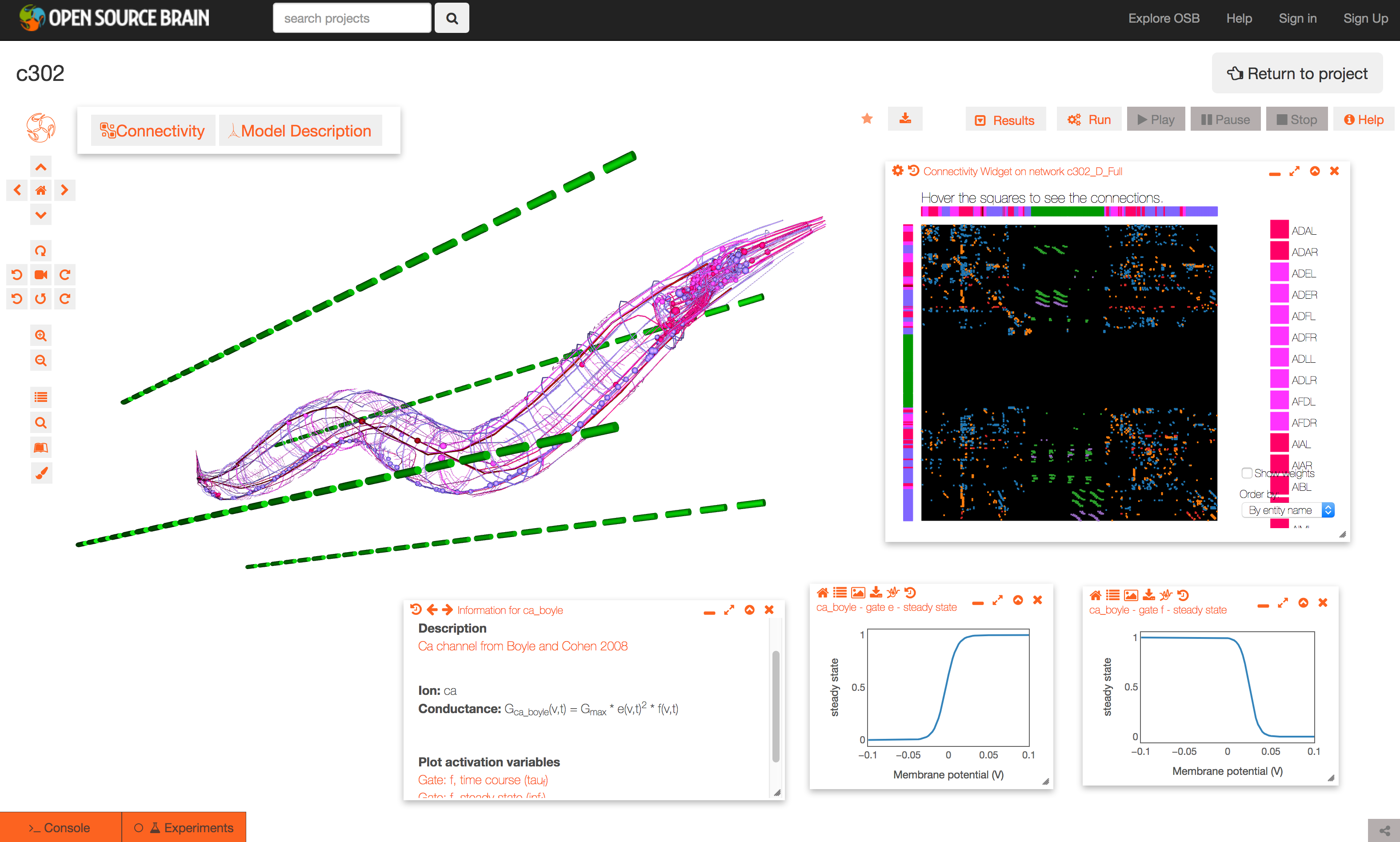
The Github repository for c302 is linked to a project on Open Source Brain: http://www.opensourcebrain.org/projects/c302. This allows exploration of the generated NeuroML 2 networks through the Geppetto enabled OSB 3D Explorer.
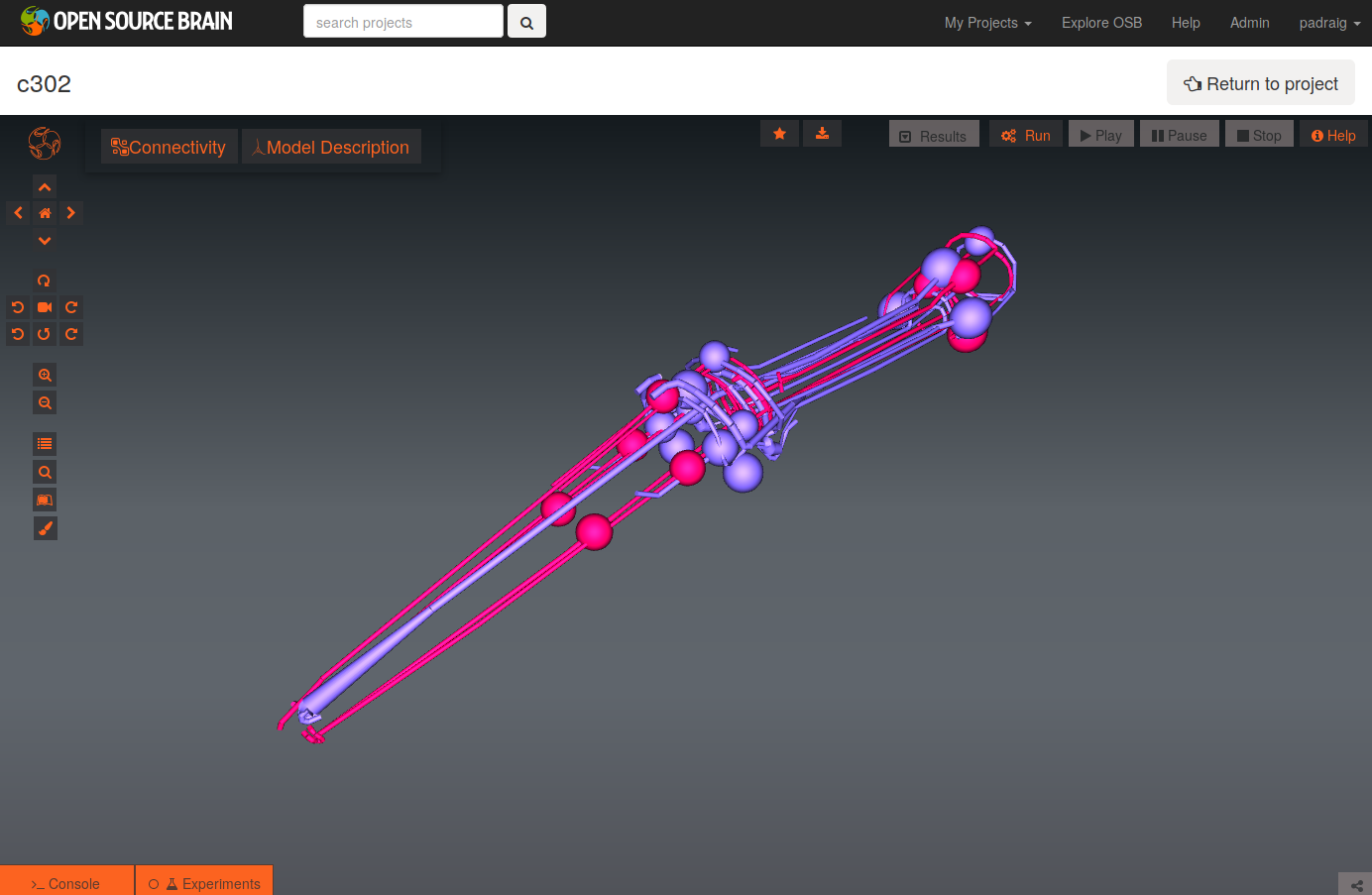
To see a list of the networks which can be visuaised, click on the More button on the top right of the page. As an example, the Pharyngeal network with parameter set D can be selected (c302_D_Pharyngeal.net.nml in the Network list, direct link here).
The initial view of the network is shown on the left below.
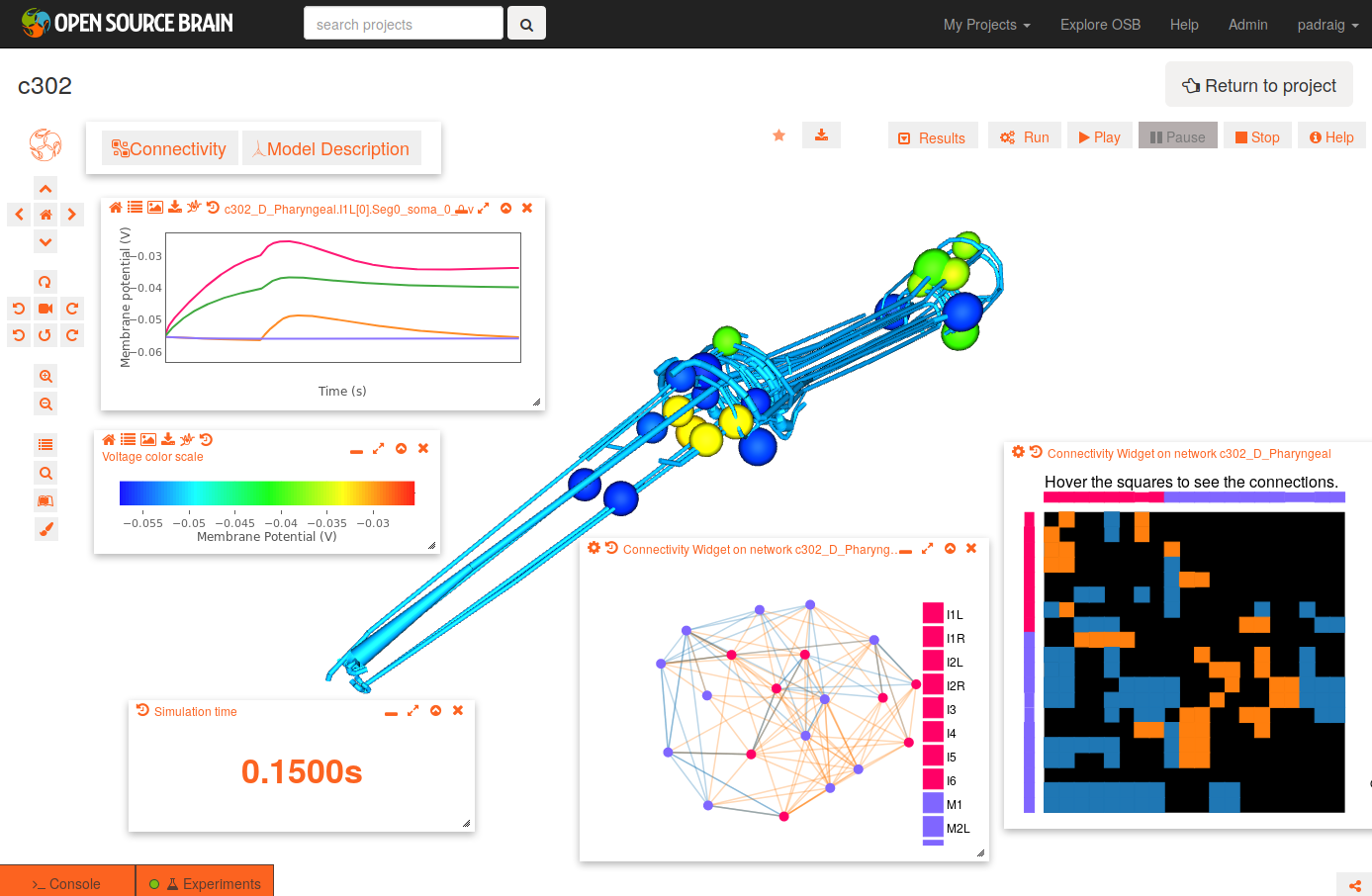
To get the view on the right:
- change the background to white by clicking the paintbrush icon (bottom icon on left)
- open the connectivity matrix (Connectivity button, top left) showing the connections present between cells in an adjacency matrix
- press Connectivity button again and select Force-directed layout
- Run a simulation of the network
- Persist project so simulations can be run (star on top middle). You will need to have signed up for OSB & logged in!
- Press Run button; set duration to 0.4 seconds; press Submit
- When dialog appears asking what to record, select all membrane potentials at somas. Simulation will be set running.
- After circle on Experiments tab has turned green, plot some of the recorded traces:
- press the Control Panel icon (four horizontal lines, forth icon from botton on left)
- show the list of state variables: x2 button on top of this dialog. Ensure target button is selected (first of four buttons on right) to only show recorded variables
- all recorded variables are shown, e.g. c302_D_Pharyngeal.I1L[0].Seg0_soma_0_0.v: membrane potential of soma in cell I1L. select which to plot with icons on right
- Replay the simulation:
- use the time varying voltage of cells to color the corresponding 3D objects: Results -> Apply voltage colouring to morphologies
- open the dialog for the simulation time: Results -> Show simulation time
- replay the simulation: Results -> Play step by step (10x)
It is also possible to view and analyse other configurations, e.g. c302_D_Full:
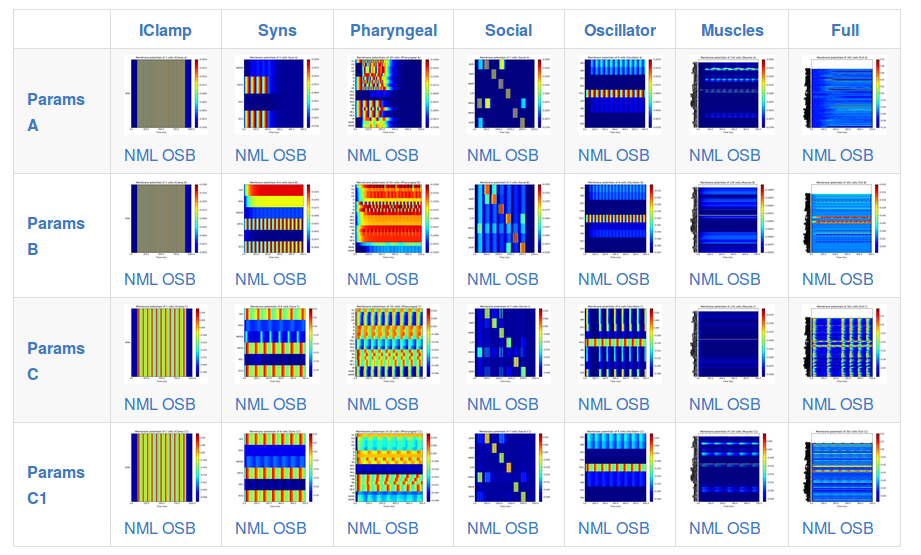
This page shows a set of generated simulations at each of the subnetwork/parameter set configurations, and provides a quick overview of the activity of the different instances of c302:
Note that c302 is still in active development and not all of the configurations are producing physiologically realistic results.
Overview of the files and packages used in the framework: